Namodenoson (CF102)
Drug Product
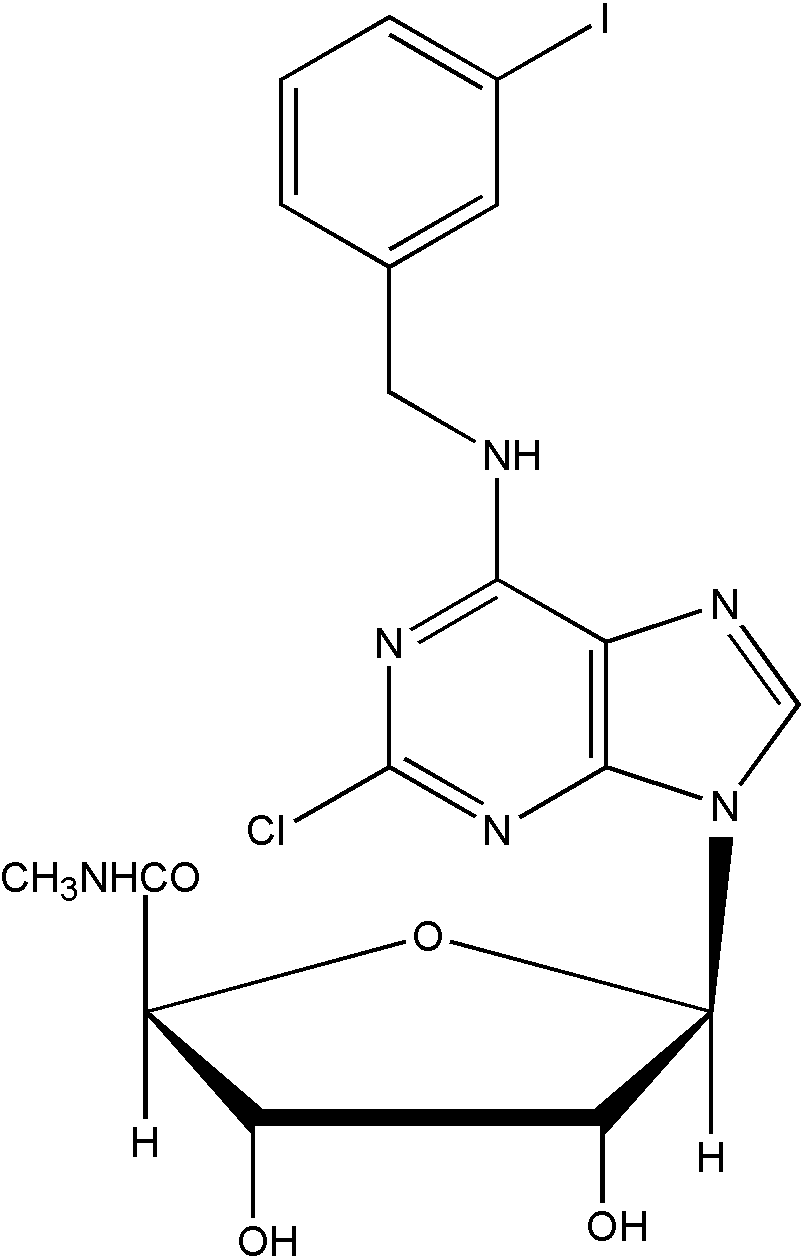
Namodenoson is an oral small molecule drug generically known as Cl-IB-MECA (2-chloro-N6-(3-iodobenzyl)-adenosine-5'- N-methyl-uronamide), a highly specific and selective agonist at the A3 adenosine receptor (A3AR).
Preclinical Pharmacology
Namodenoson has a potent anti-cancer effect, particularly against hepatocellular carcinoma, and anti-inflammatory activity demonstrated in pre-clinical animal models of liver inflammation.Mechanism of Action
Namodenoson's mechanism of action is mediated via de-regulation of the NF-κB and the Wnt signal transduction pathways, resulting in apoptosis of tumor cells. The protective effect of Namodenoson is mediated via down-regulation of the NF-kB signal transduction pathway and preventing apoptosis.Safety
The safety of Namodenoson has been demonstrated in preclinical studies, and Phase I and Phase II clinical studies demonstrating a favorable safety profile.Efficacy
- Hepatocellular Carcinoma
A Phase I/II study in hepatocellular carcinoma (HCC) successfully met its primary and secondary endpoints demonstrating initial indications for efficacy of Namodenoson. A global Phase II study treating patients with Namodenoson as a second-line therapy has recently been concluded. Although the overall survival primary endpoint failed, a robust clinical effect has been demonstrated in a sub-population of Child Pugh B7 (CPB7) patients. In an End-of-Phase II meeting with the FDA, the Company reached an agreement with the agency on a Phase III protocol in the CPB7 population. An agreement on the pivotal Phase III study has also been reached with the European Medicines Agency and the study is expected to begin. - NASH (Nonalcoholic steatohepatitis)
A Phase II study in patients with NAFLD/NASH successfully met its endpoints. A robust anti-inflammatory effect manifested by significant decrease in the liver enzymes ALT and AST and significant improvement in the positive cytokine adiponectin was recorded. A reduced liver fat content (LFC) and a reduction in % of liver fat volume was found together with a decrease in FIB-4 and FAST, non-invasive tests used as markers to exclude advanced fibrosis. Can-Fite is now preparing a Phase IIb study in this indication.
