Overview
Can-Fite has a number of drugs in various stages of research and development. The pipeline drugs are synthetic, highly specific agonists and allosteric modulators that target the A3 adenosine receptor (A3AR). All drugs are orally bioavailable with an excellent safety profile.
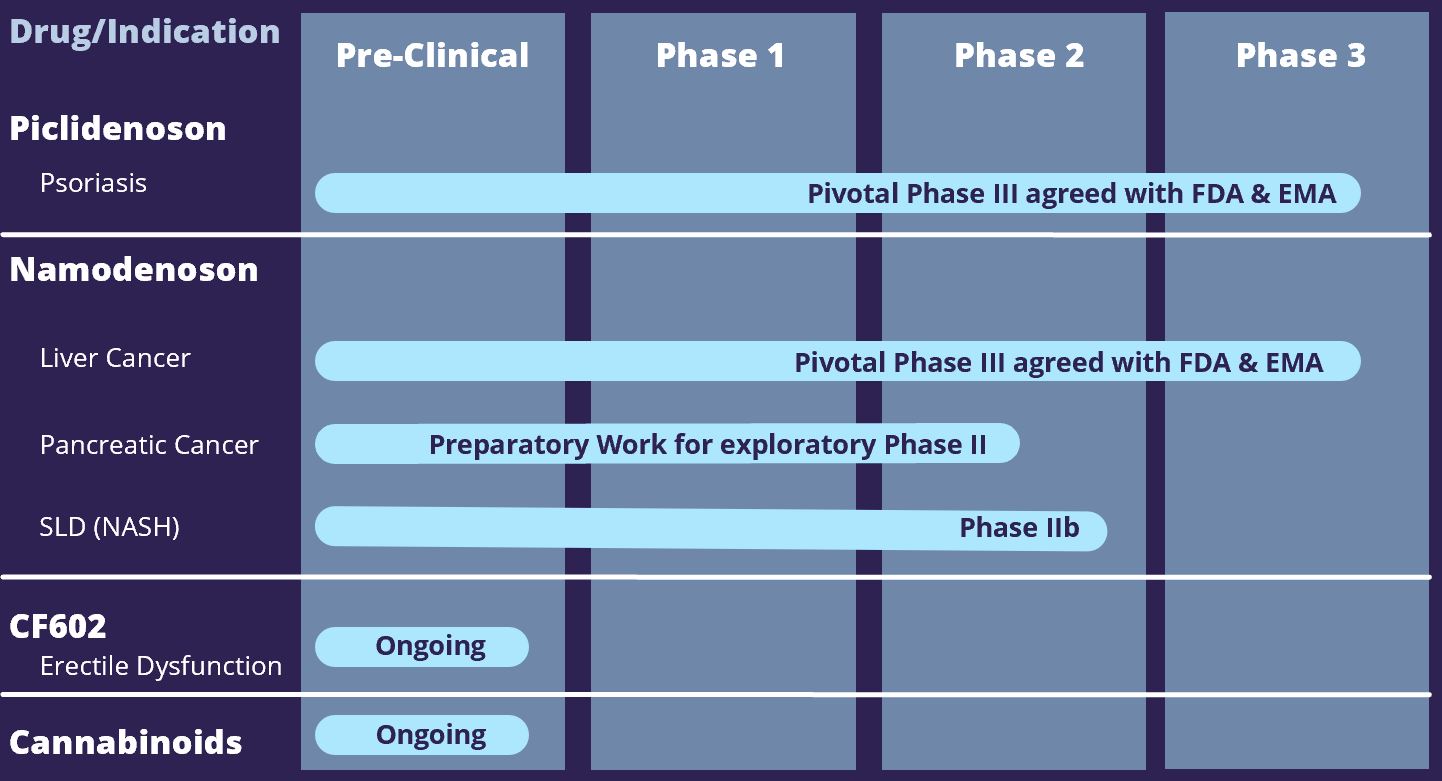
Piclidenoson (CF101), Can-Fite's lead candidate, is an oral drug that is currently in a Phase III clinical study for the treatment of psoriasis. All human clinical studies have demonstrated this drug to have an excellent safety profile. The Phase II and the Phase II/III clinical studies in psoriasis Piclidenoson showed clinical activity and safety. Can-Fite has licensed Piclidenoson for the treatment of psoriasis to Cipher Pharmaceuticals in Canada; Gebro Pharma GmbH in Spain, Switzerland & Austria; to Kyongbo in Korea; and CMS in China, Taiwan, Hong Kong, and Macao.
Namodenoson (CF102), Can-Fite's second drug candidate, is an oral drug currently being developed for the treatment of liver diseases including hepatocellular carcinoma and non-alcoholic steatohepatitis (NASH). Can-Fite has licensed Namodenoson for the treatment of liver cancer and NASH to Chong Kun Dang Pharmaceuticals in Korea and CMS in China, Taiwan, Hong Kong, and Macao.
CF602, an allosteric modulator at the A3AR, has shown proof of concept in pre-clinical pharmacology studies and is earmarked for the treatment of erectile dysfunction.
Namodenoson (CF102), Can-Fite's second drug candidate, is an oral drug currently being developed for the treatment of liver diseases including hepatocellular carcinoma and non-alcoholic steatohepatitis (NASH). Can-Fite has licensed Namodenoson for the treatment of liver cancer and NASH to Chong Kun Dang Pharmaceuticals in Korea and CMS in China, Taiwan, Hong Kong, and Macao.
CF602, an allosteric modulator at the A3AR, has shown proof of concept in pre-clinical pharmacology studies and is earmarked for the treatment of erectile dysfunction.
